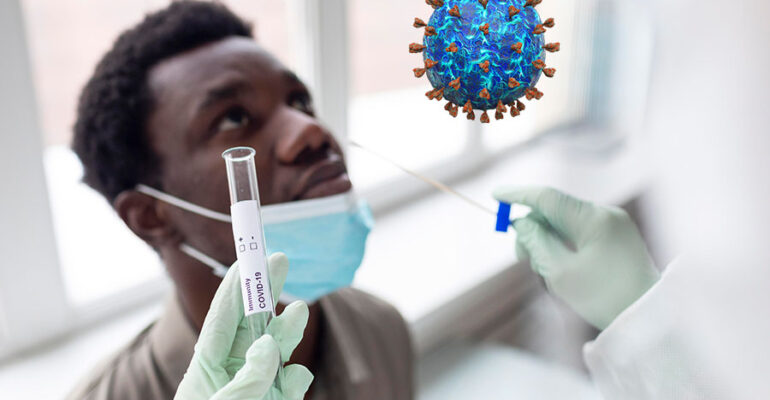
COVID-19
Coronavirus disease-2019 or COVID-19 (the disease) is caused by severe acute respiratory syndrome coronavirus-2 or SARS-CoV-2 (the virus). In 2020, it became a pandemic of (inter)national concern.
Infected people display a wide range of clinical presentation from asymptomatic to mild to moderate to severe life threatening disease. Non-vaccinated and vaccinated individuals can be infected although vaccination reduces risk of severe disease.
As a diagnostic center, Scanlab Nakuru’s role in the pandemic is in offering high quality laboratory and imaging services for screening, diagnosis and monitoring of COVID-19. We specifically offer the following services:
a) COVID-19 (SARS-CoV-2) PCR testing services.
Samples are obtained from the nose (nasopharyngeal swab) or throat (oropharyngeal swab) and tested for viral genetic material (ribonucleic acid, RNA). Results are ready within 24-72 hours after samples have been received.
As an MOH-approved laboratory, we upload test results for international travelers to the panaBIOS trusted travel database leading to generation of trusted travel (TT) codes, seals and certificates. The traveler is notified directly from panaBIOS via email. For international travelers, samples must have been collected/ received in the lab at least 48 hours prior to travel. It is imperative that the right email address is provided & that the traveler confirms that it is correctly captured by reviewing the receipt issued. Travelers should confirm the current destination-specific testing requirements with airlines, transit points & destinations. They should also ensure that information provided to the laboratory matches that on travel documents.
b) COVID-19 (SARS-CoV-2) antigen test. Rapid test results are availed on the same day of sample receipt.
c) In-house (D&D house, 5th floor, West road, Nakuru, +254717535037) & on-site (home/ institutions/ office) collection services for either COVID PCR or COVID antigen testing.
d) Customized testing panels for monitoring infected patients.
e) High resolution chest CT scan (HRCT) for evaluation of suspected COVID-19 pneumonia.
f) Training on sample management (collection, packing, storage, shipping etc.) to registered health facilities/ health care workers.